Note4Students
From UPSC perspective, the following things are important :
Prelims level: PresVu
Why in the News?
Mumbai-based Entod Pharmaceuticals has announced that the Drug Controller General of India (DCGI) has approved its new eye drop, PresVu, aimed at reducing the dependency on reading glasses for individuals with presbyopia.
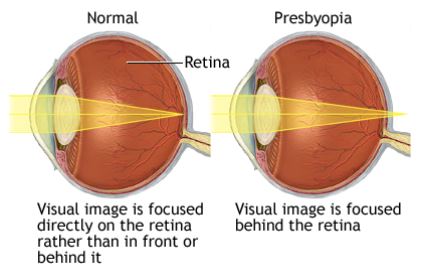
What is Presbyopia?
|
How does PresVu work?
- The active ingredient in PresVu is pilocarpine, a compound that contracts the iris muscles, controlling the size of the pupil and helping individuals focus better on nearby objects.
- PresVu also uses an advanced dynamic buffer technology to adapt to the pH levels of tears, ensuring consistent efficacy and safety for extended use over the years.
- However, PresVu’s effects are temporary, typically lasting between four to six hours, and it is prescription-only.
- PresVu should not be used by individuals with iris inflammation.
- Regular use may lead to side effects such as:
- Itching and redness
- Eyebrow pain
- Muscle spasms in the eyes
Is this a Novel Therapy?
- Although Entod claims PresVu is novel, the main compound, pilocarpine, has been available in India for decades and is commonly used as a first-line therapy for cataracts.
- Pilocarpine’s ability to temporarily improve the depth of focus has been explored in other countries, including the United States, where the FDA approved a pilocarpine eye drop for presbyopia in 2021.
- In India, the government regulates the ceiling price of pilocarpine in 4% and 2% concentrations, whereas PresVu contains 1.25%.
PYQ:[2018] Appropriate local community-level healthcare intervention is a prerequisite to achieve ‘Health for All’ in India. Explain. |
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024