From UPSC perspective, the following things are important :
Prelims level: Super-Absorbent Polymers (SAP), Polyacrylamide,Sodium Polyacrylate
Why in the News?
Understanding the absorbency of diapers through the Quantum physics of water absorption and contrasting materials that do or do not absorb water.
Absorption in Diapers: How it works?
- Absorption depends on Microscopic forces and Material properties. Water molecules are attracted to materials like cotton due to their structure.
- Cotton, a network of polymers with ions, absorbs water effectively by attracting water molecules.
- For large fluid absorption like in diapers, Super-Absorbent Polymers (SAP) are crucial.
What are Super-Absorbent Polymers (SAP)?
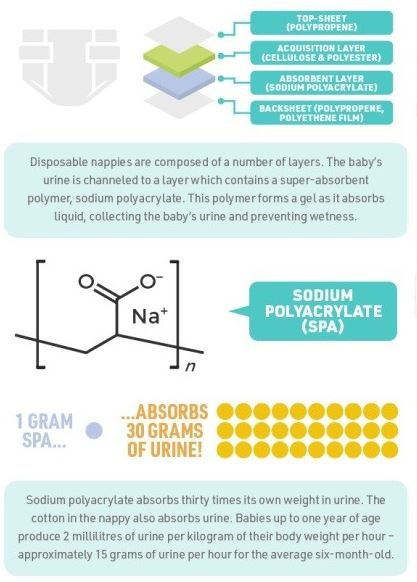
- SAPs are synthetic materials with the ability to absorb and retain large amounts of liquid relative to their own mass.
- They are commonly used in products like diapers, sanitary napkins, and other absorbent hygiene products.
- SAPs are typically cross-linked polymers, meaning their molecules are bonded in a way that creates a network capable of absorbing water molecules.
Examples:
- Sodium Polyacrylate: This is one of the most common types of SAP used in diapers. It forms a gel-like substance when it absorbs liquid.
- Polyacrylamide: Another type of SAP used in various applications, including agriculture and wastewater treatment, due to its high water-absorbing capacity.
Quantum Physics Insight of SAP
Quantum physics plays a fundamental role in understanding the behaviour of super-absorbent polymers (SAPs), particularly in how they interact with water molecules at the atomic level:
- Electron Sharing: SAPs contain ions like sodium, which have a strong affinity for water molecules. This attraction is based on the principles of quantum physics, where atoms like sodium and oxygen prefer to share electrons to achieve stability. This shared electron arrangement allows water molecules to bond with the ions in SAPs, facilitating the absorption process.
- Quantum Mechanical Properties: At the quantum level, electrons behave as waves and can exist in shared states between atoms. This phenomenon allows for the formation of stable bonds between water molecules and SAP ions, enhancing the SAP’s ability to absorb large amounts of liquid.
- Energy States: Quantum physics explains how SAPs manage energy states during absorption. As water enters the SAP, energy is released due to changes in the electron configurations and bonding energies of the ions involved. This process is crucial for maintaining the gel-like structure of the SAP and preventing leakage.
PYQ:[2022] Which one of the following is the context in which the term “qubit” is mentioned? (a) Cloud Services (b) Quantum Computing (c) Visible Light Communication Technologies (d) Wireless Communication Technologies |
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
